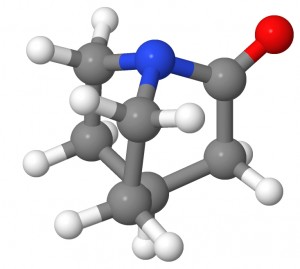
 The amide bond is the essential structural motif of the protein backbone. The hydrolysis reaction of amides, often used as a model for the cleavage of peptide bonds, is thus of primary concern for living systems. The hydrolysis of non-activated amides is very slow and in most cases undetectable. An amide bond’s stability is ascribed to its partial double bond character, caused by the delocalization between the nitrogen lone pair and the π* orbital of CO bond. As a consequence, amides show a characteristic short C-N bond length and a rigid planar conformation. This stability also has important chemical consequences, a low reactivity toward nucleophilic attacks on the carbon and important basicity shifts of the nitrogen with respect to amines. (more…)
The amide bond is the essential structural motif of the protein backbone. The hydrolysis reaction of amides, often used as a model for the cleavage of peptide bonds, is thus of primary concern for living systems. The hydrolysis of non-activated amides is very slow and in most cases undetectable. An amide bond’s stability is ascribed to its partial double bond character, caused by the delocalization between the nitrogen lone pair and the π* orbital of CO bond. As a consequence, amides show a characteristic short C-N bond length and a rigid planar conformation. This stability also has important chemical consequences, a low reactivity toward nucleophilic attacks on the carbon and important basicity shifts of the nitrogen with respect to amines. (more…)
Kimika Teorikoa
Euskal Herriko Unibertsitatea


