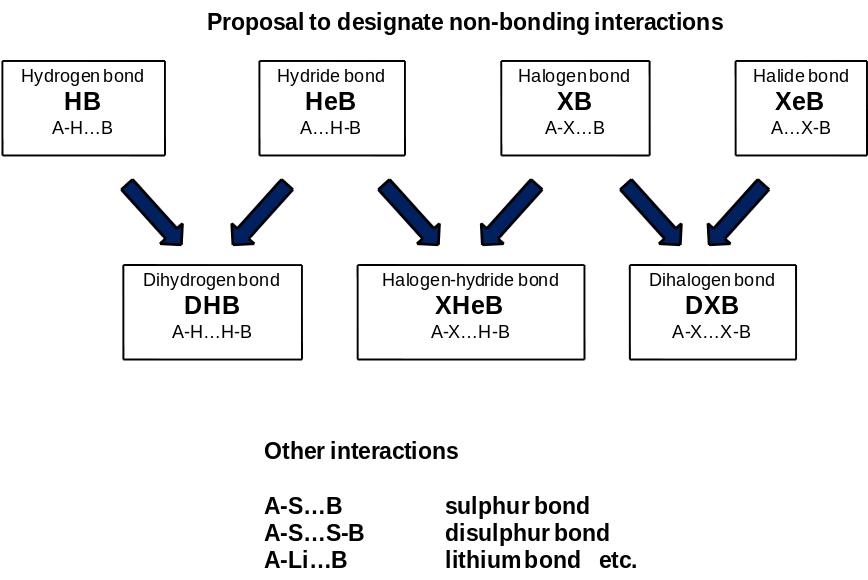
There are various non-bonding interactions which are important in numerous chemical processes and phenomena.i These interactions may be treated as counterparts or competitors of hydrogen bond. It was found that the common characteristic for them, including hydrogen bond, is the electron charge transfer from the Lewis base to the Lewis acid and that the amount of this transfer corresponds roughly to the strength of the interaction.ii The following non-bonding interactions may be mentioned; the dihydrogen bond, the halogen bond, the agnostic bond, the hydride bond and others. In recent years the σ-hole concept was introduced and developed and it was applied to non-bonding interactions.
The non-bonding interactions possess the similar characteristics as the hydrogen bond; and they may be explained in terms of Bent´s rule.iii For example, if one considers the C-Cl…B halogen bond thus its formation leads to the transfer of electrons from the Lewis base (B) into the antibonding C-Cl orbital of the Lewis acid sub-unit of the complex. This is connected with the increase of the positive charge on the Cl atom and the increase of the negative charge on C atom. The increase of the s-character in the C orbital of the C-Cl bond is observed. All these changes are in agreement with Bent´s rule since the complexation leads to the increase of the electropositive character of the Cl atom. Figure 1 illustrates these changes for the complexes of F3CCl. This is important that numerous non-bonding interactions may be treated as a first stage of biochemical processes.
i Hobza, P.; Müller-Dethlefs, K. Non-Covalent Interactions, Theory and Experiment, Royal Society of Chemistry, Thomas Graham House, Science Park, Milton Road, Cambridge, 2010.