Áreas de Interés
The development of new methods for the construction of carbon-carbon and carbon-heteroatom bonds is a key aspect in chemical synthesis. Today the increasing societal concerns about minimum production of waste materials, suitability of less toxic, purer materials (drugs), saving cost and energy, etc., demand improved, more atom economic synthetic technologies. The use of catalysts to promote reactions efficiently and selectively is the best known approach to such a goal, something nature is doing constantly by using enzymes.General application of enzymes to chemical synthesis is still limited due to their high specificity and low chemical stability. Therefore the design of new catalytic systems, that may include the design of new catalysts and/or a combination of known catalysts with better achiral templates, is of crucial importance to meet the criteria of chemo-, regio-, and stereoselectivity required.
On the other hand, mimicking bio-molecules such as peptides provides new molecular entities that may serve for better understanding their biochemical behaviour and, eventually, for the discovery of new drug leads. We are interested in the construction of new peptidomimetics through structural modulation by using logically pre-designed strategies.
The following topics are currently under investigation in our laboratories:
- Asymmetric catalysis.
- C-C and C-heteroatom bond formation.
- New molecular entities with biological properties.
Some recent contributions are illustrated below:
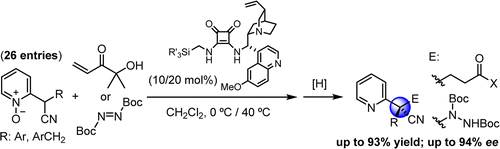
- "Base-Catalyzed Asymmetric α‑Functionalization of 2‑(Cyanomethyl)azaarene N‑Oxides Leading to Quaternary Stereocenters". J. Izquierdo, A. Landa, I. Bastida, R. López, M. Oiarbide, C. Palomo. J. Am. Chem. Soc. 2016, 138, 3282–3285.
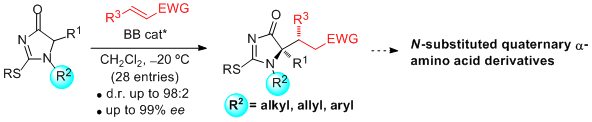
- "1H-Imidazol-4(5H)-ones are introduced as novel nucleophilic α-amino acid equivalents in asymmetric synthesis. These compounds not only allow highly efficient construction of tetrasubstituted stereogenic centers, but unlike hitherto known templates, provide direct access to N-substituted (alkyl, allyl, aryl) α-amino acid derivatives". J. Etxabe, J. Izquierdo, A. Landa, M. Oiarbide, C. Palomo. Angew. Chem. Int. Ed. 2015, 54, 6883 – 6886.
- "Enantioselective Construction of Tetrasubstituted Stereogenic Carbons through Brønsted Base Catalyzed Michael Reactions: α′-Hydroxy Enones as Key Enoate Equivalent". E. Badiola, B. Fiser, E. Gomez-Bengoa, A. Mielgo, I. Olaizola, I. Urruzuno, J. M. García, J. M. Odriozola, J. Razkin, M. Oiarbide, C. Palomo. J. Am. Chem. Soc. 2014, 136, 17869 – 17881.
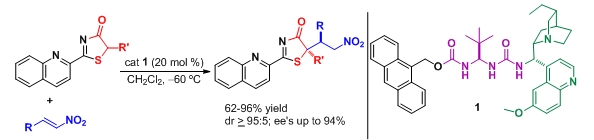
- "Catalytic Enantioselective Synthesis of Tertiary Thiols From 5H-Thiazol-4-ones and Nitroolefins Mediated By a Bifunctional Ureidopeptide-Based Brønsted Base Catalyst". S. Diosdado, J. Etxabe, J. Izquierdo, A. Landa, A. Mielgo, I. Olaizola, R. López, C. Palomo. Angew. Chem. Int. Ed. 2013, 52, 11846 –11851.
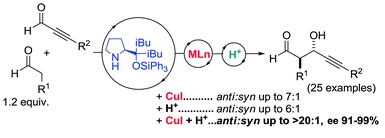
- "Asymmetric synthesis of propargylic alcohols via aldol reaction of aldehydes with ynals promoted by propinol ether-transition metal-Bronsted acid cooperative catalysis". E. Gómez-Bengoa, J. M. García, S. Jiménez, I. Lapuerta, A. Mielgo, J. M. Odriozola, I. Otazo, J. Razkin, I. Urruzuno, S. Vera, M. Oiarbide, C. Palomo. Chem. Sci., 2013, 4, 3198-3204.
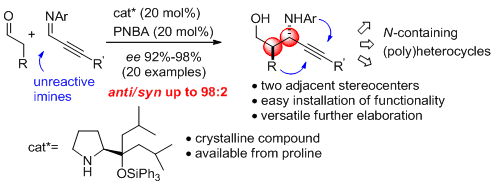
- Optically active versatile propargylic aminoalcohols are affordable via the first enamine mediated anti-selective and highly enantioselective Mannich reaction of aldehydes with unactivated imines. "Combined α,α-dialkylprolinol ether/Bronsted acid promotes Mannich reactionsof aldehydes with unactivated imines. An entry to anti-configured propargylic amino alcohols" E. Goméz-Bengoa, J. Jiménez, I. Lapuerta, A. Mielgo, M. Oiarbide, I. Otazo, I. Velilla, S. Vera, C. Palomo. Chem. Sci. 2012, 3, 2949-2957.
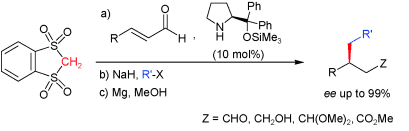
- Cyclic geminal bis(sulfone)s are able to engage in amine-catalyzed C-C bond-forming reactions involving both 1,2- and 1,4-type acceptors. With α,β-unsaturated aldehydes as acceptors and chiral pyrrolidines as catalysts, very high selectivity and generality are attained. Upon consecutive sulfone a-alkylation and desulfonation of the resulting adducts, useful building blocks are accesible with broad functional-group tolerance and high ee. "Catalytic Conjugate Additions of Geminal Bis (sulfone)s: Expanding the Chemistry of Sulfones as Simple Alkyl Anion Equivalents" A. Landa, A. Puente, J. I. Santos, S. Vera, M. Oiarbide, C. Palomo. Chem. Eur. J. 2009, 15, 11954-11962.
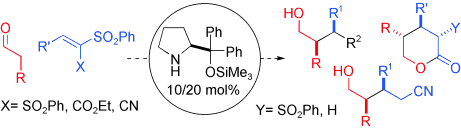
- Joined together, organocatalysts aldehydes and sulfones: A diaryl prolinol silyl ether was found to catalyse efficiently and enantioselectively the conjugate addition of aldehydes to vinyl sulfones. The ample synthetic utility of the resulting adducts is illustrated. "Highly Enantioselective Conjugate Addition of Aldehydes to Vinyl Sulfones" A. Landa, M. Maestro, C. Masdeu, A. Puente, S. Vera, M. Oiarbide, C. Palomo. Chem. Eur. J. 2009, 15, 1562-1565.
- A step further in the enantioselective Cu-catalyzed conjugate addition of R2Zn reagents to enoyl systems is taken by using sterically encumbered α´-oxy enone templates(see scheme). The method constitutes an advanced entry to enantioenriched β-branched carboxylic acids and aldehydes with a broad R2Zn and functional group tolerance. "Copper-Catalyzed Enantioselective Conjugate Addition of Dialkylzinc Reagents to α´-Oxy Enones" J. M. García, A. González, B. G. Kardak, J. M. Odriozola, M. Oiarbide, J. Razkin, C. Palomo. Chem. Eur. J. 2008, 14, 8768-5771.
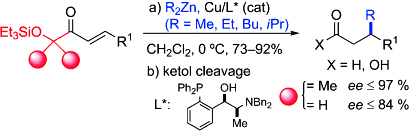
- Discriminating reactions: Whilst no self-aldol reaction is observed, catalyst I promotes the Michael addition of aldehydes to nitroalkenes with the lowest catalyst loading and aldehyde ratio until now reported for this type of reaction. "Highly Efficient Asymmetric Michael Addition of Aldehydes to Nitroalkenes Catalyzed by a Simple Trans-4-hydroxyprolylamide". C. Palomo, S. Vera, A. Mielgo, E. Gómez-Bengoa. Angew. Chem. Int. Ed. 2006, 45, 5984-5987.
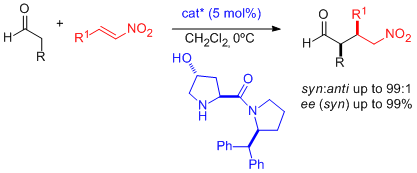
- A combination of zinc triflate, an amine base, and (-)-N-methylephedrine (NME), which can be easily recovered and reused, leads to high enantioselectivities in the aza-Henry reaction of N-Boc-protected aldimines and nitromethane (see scheme; Boc=tert-butyloxycarbonyl). "Enantioselective Aza-Henry Reactions Assisted by Zn(II) and N-Methyl Ephedrine". C. Palomo, M. Oiarbide, R. Halder, A. Laso, R. López. Angew. Chem. Int. Ed. 2006, 45, 117-120.
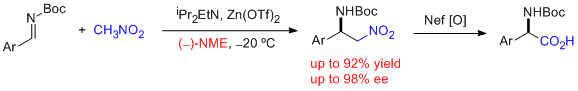
- In situ generated azomethines from readily available precursors react with nitromethane in the presence of 120 mol % of CsOHxH2O and 12 mol % of quinine- and cinchonidine-derived quaternary ammonium chlorides to provide the corresponding aza-Henry adducts in good yields and very high selectivities. It represents the first general enantioselective aza-Henry method for azomethines derived from enolizable aldehydes, giving rise to ee's above 94%. In addition, the reactions with nitroethane afforded high diastereo- and enantioselectivities (syn:anti up to 95:5; up to 98% ee for syn). "Catalytic Enantioselective Aza-Henry Reaction with Broad Substrate Scope" C. Palomo, M. Oiarbide, A. Laso, R. López. J. Am. Chem. Soc. 2005, 127, 17622-17623.
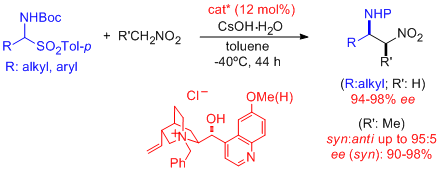
- Excellent combined levels of regio-, endo/exo-, diastereo-, and/or enantioselectivity are provided in the Cu-catalyzed cycloaddition of nitrones with α'-hydroxy enones. The resulting adducts are amenable for easy chemical elaboration to the corresponding isoxazolidines bearing aldehyde, ketone, and carboxylic acid functions. "Lewis Acid Catalyzed Asymmetric Cycloadditions of Nitrones: α'-Hydroxi Enones as Efficient Reaction Partners" C. Palomo, M. Oiarbide, E. Arceo, J. M. García, R. López, A. González, A. Linden. Angew. Chem. Int. Ed. 2005, 44, 6187-6190.
- A simple assembly of commercially available materials, namely, zinc triflate (Zn(OTf)2), a tertiary amine base (iPr2EtN), and reusable (+)-N-methylephedrine, effectively activates the Henry reaction between aldehydes and nitromethane (see scheme). The catalytic system can be used in substoichiometric quantities and leads to good yields and enantioselectivities. "Enantioselective Henry Reactions under Dual Lewis Acid/Amine Catalysis Using Chiral Amino Alcohol Ligands" C. Palomo, M. Oiarbide, A. Laso. Angew. Chem. Int. Ed. 2005, 44, 3881-3884.
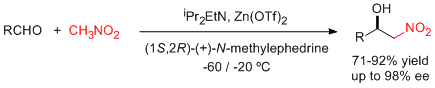
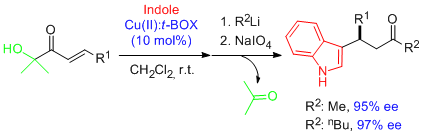
- Remarkably high and regular enantioselectivities are obtained in Friedel-Crafts alkylation reactions involving α'-hydroxy enone templates and Cu(II)-bis(oxazoline) complexes as catalysts. The simple elaboration of adducts provides a route to enantioenriched aldehydes, carboxylic acids and ketones containing the pyrrole and indole frameworks. "Highly Enantioselective Friedel-Crafts Alkylations of Pyrroles and Indoles with α-Hydroxy Enones under Cu(II)-Simple Bis(oxazoline) Catalysis". C. Palomo, M. Oiarbide, B. G. Kardak, J. M. García, A. Linden. J. Am. Chem. Soc. 2005, 127, 4154-4155.
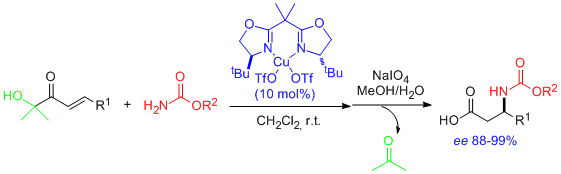
- Catalytic, asymmetric conjugate addition of carbamates to enoyl systems has been realized for the first time, providing a two-step access to virtually enantiopure N-protected β-amino acids. "Catalytic Enantioselective Conjugate Addition of Carbamates". C. Palomo, M. Oiarbide, R. Halder, M. Kelso, E. Gómez-Bengoa, and J. M. García. J. Am. Chem. Soc. 2004, 126, 9188-9189.
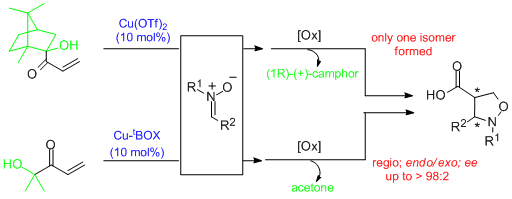
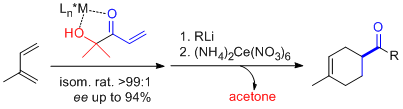
- α'-Hydroxy enones react with dienes in the presence of (S,S)-[Cu(tBu-box)](OTf)2 or (S,S)-[Cu(tBu-box)](SbF6)2 (2 to 10 mol%) to afford the corresponding Diels-Alder adducts in high yield and selectivity. Isomeric ratios (regioselectivity, endo/exo or cis/trans) of up to >99:1 and ee values of up to >99% are obtained. Significantly, difficult dienes such as isoprene, 2,3-dimethyl butadiene and piperylene behave satisfactorily. Subsequent oxidative cleavage of the ketol in the resulting cycloadducts by treatment with cerium ammonium nitrate (CAN) yields the corresponding enantiopure carboxylic acids. Alternatively, carbonyl addition and subsequent diol cleavage with CAN produces the corresponding ketone adducts. "α-Hydroxy Enones as Achiral Templates for Lewis Acid-Catalyzed Enantioselective Diels-Alder Reactions" C. Palomo, M. Oiarbide, J. M. García, A. González, E. Arceo. J. Am. Chem. Soc. 2003, 125, 13942-13943.
- The inclusion of a β-lactam ring into a peptide backbone leads to the formation of a new peptide product that adopts a very stable type II β-turn motif in solution, even in dimethylsulfoxide as the solvent. The nature of the N- and C- terminal residues appears to have little effect on the betagenic ability of the central - (β-lactam) - (α-AA) - segment and, as a result, R1 and R2 groups can be designed specifically as recognition elements. Development of a New Family of Conformationally Restricted Peptides as Potent Nucleators of β-Turns. Design, Synthesis, Structure, and Biological Evaluation of a β-Lactam Peptide Analogue of Melanostatin. C. Palomo, J. M. Aizpurua, A. Benito, J. I. Miranda, R. M. Fratila, C. Matute, M. Domercq, F. Gago, S. Martin-Santamaria, A. Linden. J. Am. Chem. Soc. 2003, 125, 16243-16260.
- A new type of sugar-amino acid hybrid, which is comprised of a sugar unit (gluco-, galacto-, or mannopyranose) linked through a C-glycosydic linkage to the β-position of an α-unsubstituted β-amino acid unit, is presented. It is hypothesized that these new compounds, or the oligomeric peptides derived therefrom, might possess the structural features of β-amino acid oligomers and the chemical and enzymatic resistance of C-glycosides to hydrolysis. Design and Synthesis of a Novel Class of Sugar-Peptide Hybrids: C- Linked Glyco β-Amino Acids Through a Stereoselective Acetate Mannich Reaction as The Key Strategic Element C. Palomo, M. Oiarbide, A. Landa, M. C. Gonzalez Rego, J. M. Garcia, A. Gonzalez, J. M. Odriozola, M. Martín Pastor, A. Linden. J. Am. Chem. Soc. 2002, 124,8637-8643.
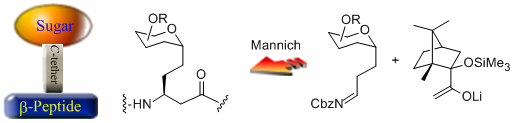
- A Chiral Acrylate Equivalent for Metal-Free Diels-Alder Reaction: Endo-2-Acryloylisoborneol C. Palomo, M. Oiarbide, J. M. Garcia, A. Gonzalez, A. Lecumberri, A. Linden. J. Am. Chem. Soc. 2002, 124, 10288-10289.
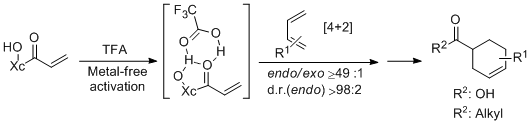
- "A Chiral Acrylate Equivalent for Metal-Free Diels-Alder Reaction: Endo-2-Acryloylisoborneol". C. Palomo, M. Oiarbide, J. M: Garcia, A. Gonzalez, A. Lecumberri, A. Linden. J. Am. Chem. Soc. 2002, 124, 10288-10289.
