Since magnesium alloys are degradable, they could provide an alternative to the metals traditionally used as prostheses, connecting parts to heal bones or as stents for cardiovascular problems. A study by the UPV/EHU Faculty of Engineering in Bilbao has succeeded in making progress on one of the weak points of the new material: its degradation rate has been adjusted by applying a calcium phosphate coating to the surface by means of electrodeposition, and the thickness of the layer has been accurately adjusted.
Prostheses with controlled degradation rate
A study by the UPV/EHU-University of the Basque Country has improved magnesium alloys for degradable prostheses, thus rendering the need for additional surgery unnecessary
First publication date: 22/06/2017

Bimetals have long been used in medicine, mainly in prostheses, but also in connecting parts to heal bones or in stents used to solve cardiovascular problems, among other things. The metals most used traditionally —stainless steel and titanium alloys— offer advantages, such as their resistance to corrosion in the physiological environment, but also drawbacks, such as the reduction in bone density close to the prosthesis, which leads to a loss of bone resistance. Furthermore, on many occasions additional surgery has to be performed to remove the material once it has fulfilled its function.
To solve these problems, many pieces of research are being conducted on other materials, such as the group comprising magnesium and its alloys. "What makes this material particularly attractive is its capacity to dissolve in the physiological environment, in other words, it would gradually dissolve until, once its mission has been completed, it is expelled from the body naturally via the urine," explained Nuria Monasterio, author of the study carried out at the UPV/EHU Faculty of Engineering in Bilbao. This would obviate the need for patients to undergo further surgery. Another strong point in the new material is that it prevents the loss of localized bone density caused by other more resistant materials. "As it is also a material in plentiful supply in the earth's crust, the cost of the raw material is reasonable, even though the processing of it requires certain precautions that raise the cost of manufacturing the alloys. So its final cost would be halfway between those of stainless steel and titanium alloys".
However, this metal also poses challenges since "its dissolution rate is higher than the desired one. It dissolves before it has fulfilled its function; so the challenge is to extend its life so that in some way it can be adjusted to fit the application," confirmed Monasterio.
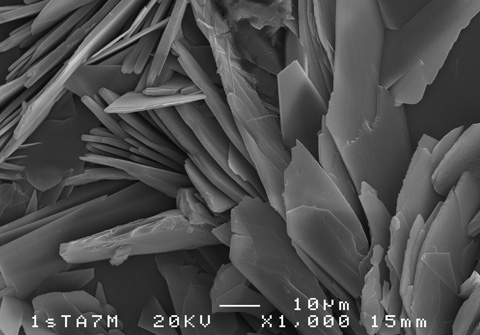
Calcium phosphate coating
There are various techniques to try to extend the life of magnesium alloys; this research by the UPV/EHU has opted to coat the material with calcium phosphate, although "the function of the calcium phosphate is not just to extend the life of the magnesium itself. It is also about the human body tolerating it better and increasing the rate of generation of the adjacent tissue, a dual function that involves extending the life of the material and achieving better integration. One has to bear in mind, firstly, that it is the main component of bones and, secondly, it has been shown to encourage the growth of the surrounding tissue," she pointed out.
Electrodeposition was used to get the calcium phosphate layer to adhere to the surface of the metal. "What we were after was to get a uniform coating that would not become detached; we also wanted to be able to vary its thickness effectively. To do this, a range of electrical variables were explored so that the thicknesses could be adapted in line with what was required by specific applications". And the result turned out to be more than satisfactory: "besides validating the method used, it has been possible to accurately regulate the quality and thickness of the layer," stressed Nuria Monasterio.
The UPV/EHU researcher mentioned various challenges with the future in mind. "We have managed to fine-tune the electrolytic system so we are now aiming to try it out on other bimetals. We are also working on manufacturing magnesium alloys of compositions that do not pose any risk, because the magnesium alloy used in this research contains aluminium, a metal that is a health hazard".
Bibliographic reference
- Analysis of electrodeposition processes to obtain calcium phosphate layer on AZ31 alloy
- Surface and Coatings Technology (2017: Volume 319: Pages 12-22)
- DOI: 10.1016/j.surfcoat.2017.03.060