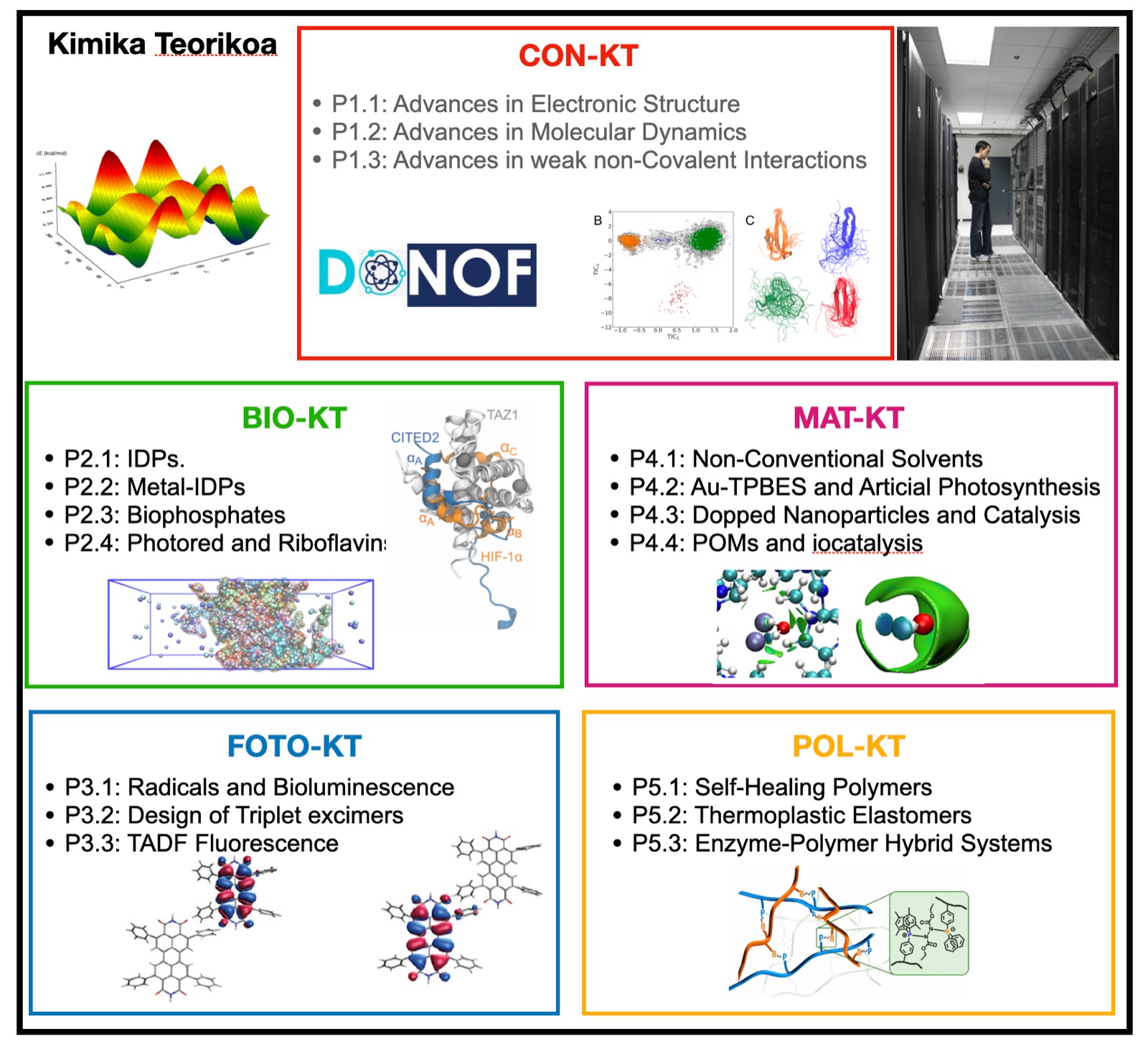
Research Lines
Publications
Lew-Yee, Juan Felipe Huan; Bonfil-Rivera, Iván Alejandro; Piris, Mario; del Campo, Jorge M.
Excited States by Coupling Piris Natural Orbital Functionals with the Extended Random-Phase Approximation Journal Article
In: J. Chem. Theory Comput., vol. 20, no. 5, pp. 2140–2151, 2024, ISSN: 1549-9626.
Links | BibTeX | Tags: Computer Science Applications, Physical and Theoretical Chemistry
@article{Lew-Yee2024,
title = {Excited States by Coupling Piris Natural Orbital Functionals with the Extended Random-Phase Approximation},
author = {Juan Felipe Huan Lew-Yee and Iván Alejandro Bonfil-Rivera and Mario Piris and Jorge M. del Campo},
doi = {10.1021/acs.jctc.3c01194},
issn = {1549-9626},
year = {2024},
date = {2024-03-12},
journal = {J. Chem. Theory Comput.},
volume = {20},
number = {5},
pages = {2140--2151},
publisher = {American Chemical Society (ACS)},
keywords = {Computer Science Applications, Physical and Theoretical Chemistry},
pubstate = {published},
tppubtype = {article}
}
Xu, Xiang; Soriano-Agueda, Luis; López, Xabier; Ramos-Cordoba, Eloy; Matito, Eduard
All-Purpose Measure of Electron Correlation for Multireference Diagnostics Journal Article
In: J. Chem. Theory Comput., vol. 20, no. 2, pp. 721–727, 2023, ISSN: 1549-9626.
Links | BibTeX | Tags: Computer Science Applications, Physical and Theoretical Chemistry
@article{Xu2023,
title = {All-Purpose Measure of Electron Correlation for Multireference Diagnostics},
author = {Xiang Xu and Luis Soriano-Agueda and Xabier López and Eloy Ramos-Cordoba and Eduard Matito},
doi = {10.1021/acs.jctc.3c01073},
issn = {1549-9626},
year = {2023},
date = {2023-12-29},
urldate = {2024-01-23},
journal = {J. Chem. Theory Comput.},
volume = {20},
number = {2},
pages = {721--727},
publisher = {American Chemical Society (ACS)},
keywords = {Computer Science Applications, Physical and Theoretical Chemistry},
pubstate = {published},
tppubtype = {article}
}
Grabowski, Sławomir J.
In: IJMS, vol. 24, no. 15, 2023, ISSN: 1422-0067.
Abstract | Links | BibTeX | Tags: Catalysis, Computer Science Applications, General Medicine, Inorganic Chemistry, Molecular Biology, Organic Chemistry, Physical and Theoretical Chemistry, Spectroscopy
@article{Grabowski2023,
title = {Ga···C Triel Bonds—Why They Are Not Strong Enough to Change Trigonal Configuration into Tetrahedral One: DFT Calculations on Dimers That Occur in Crystal Structures},
author = {Sławomir J. Grabowski},
doi = {10.3390/ijms241512212},
issn = {1422-0067},
journal = {IJMS},
volume = {24},
number = {15},
publisher = {MDPI AG},
abstract = {Structures characterized by the trigonal coordination of the gallium center that interacts with electron rich carbon sites are described. These interactions may be classified as Ga···C triel bonds. Their properties are analyzed in this study since these interactions may be important in numerous chemical processes including catalytical activities; additionally, geometrical parameters of corresponding species are described. The Ga···C triel bonds discussed here, categorized also as the π-hole bonds, do not change the trigonal configuration of the gallium center into the tetrahedral one despite total interactions in dimers being strong; however, the main contribution to the stabilization of corresponding structures comes from the electrostatic forces. The systems analyzed theoretically here come from crystal structures since the Cambridge Structural Database, CSD, search was performed to find structures where the gallium center linked to CC bonds of Lewis base units occurs. The majority structures found in CSD are characterized by parallel, stacking-like arrangements of species containing the Ga-centers. The theoretical results show that interactions within dimers are not classified as the three-centers links as in a case of typical hydrogen bonds and numerous other interactions. The total interactions in dimers analyzed here consist of several local intermolecular atom–atom interactions; these are mainly the Ga···C links. The DFT results are supported in this study by calculations with the use of the quantum theory of atoms in molecules, QTAIM, the natural bond orbital, NBO, and the energy decomposition analysis, EDA, approaches. },
keywords = {Catalysis, Computer Science Applications, General Medicine, Inorganic Chemistry, Molecular Biology, Organic Chemistry, Physical and Theoretical Chemistry, Spectroscopy},
pubstate = {published},
tppubtype = {article}
}



















